INDICATIONS FOR USE
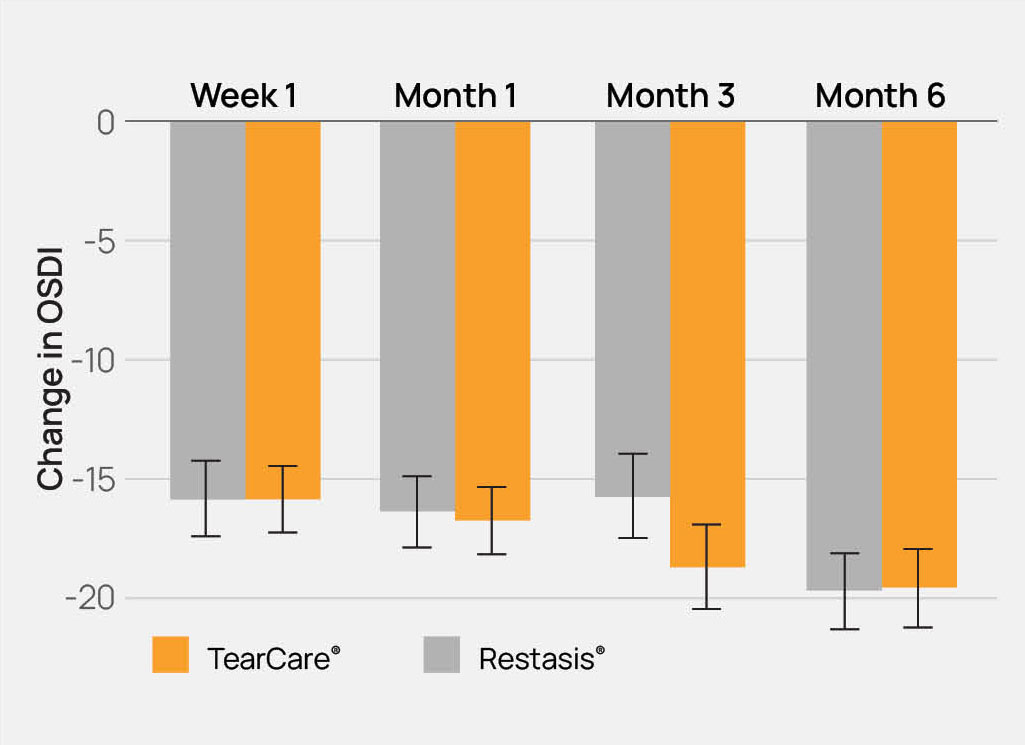
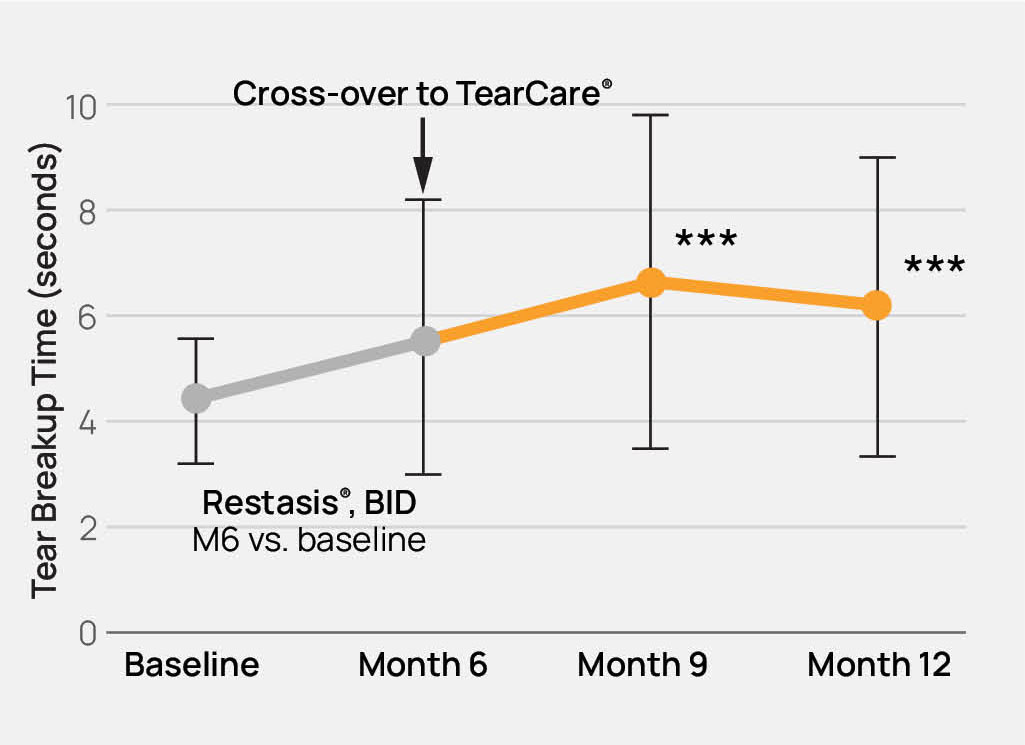
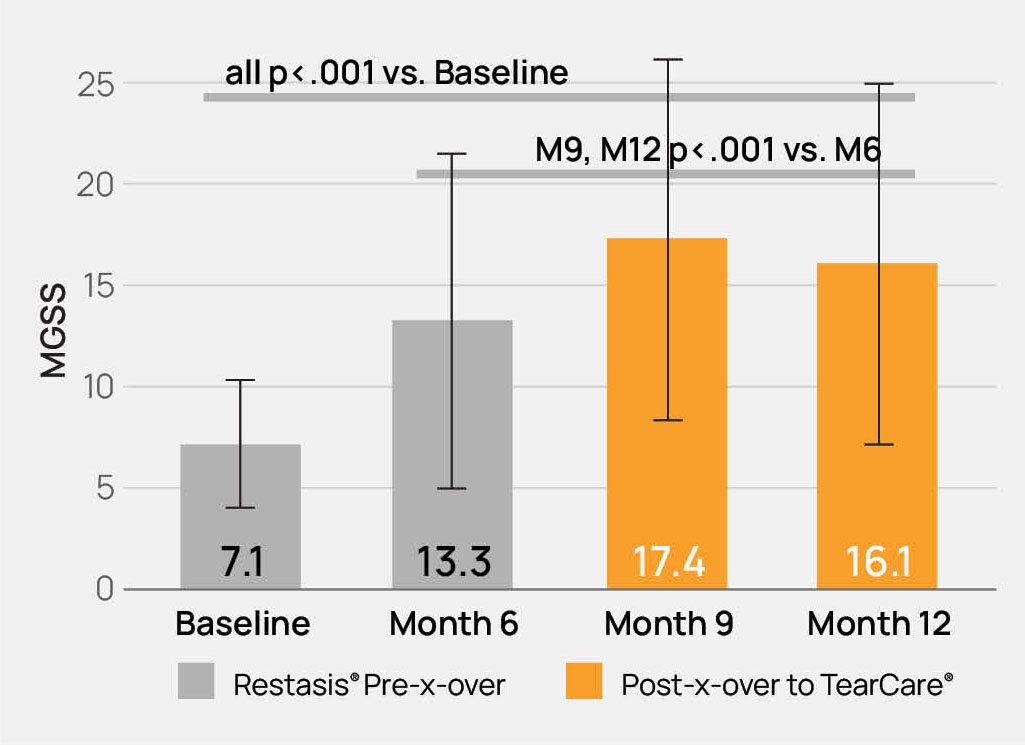
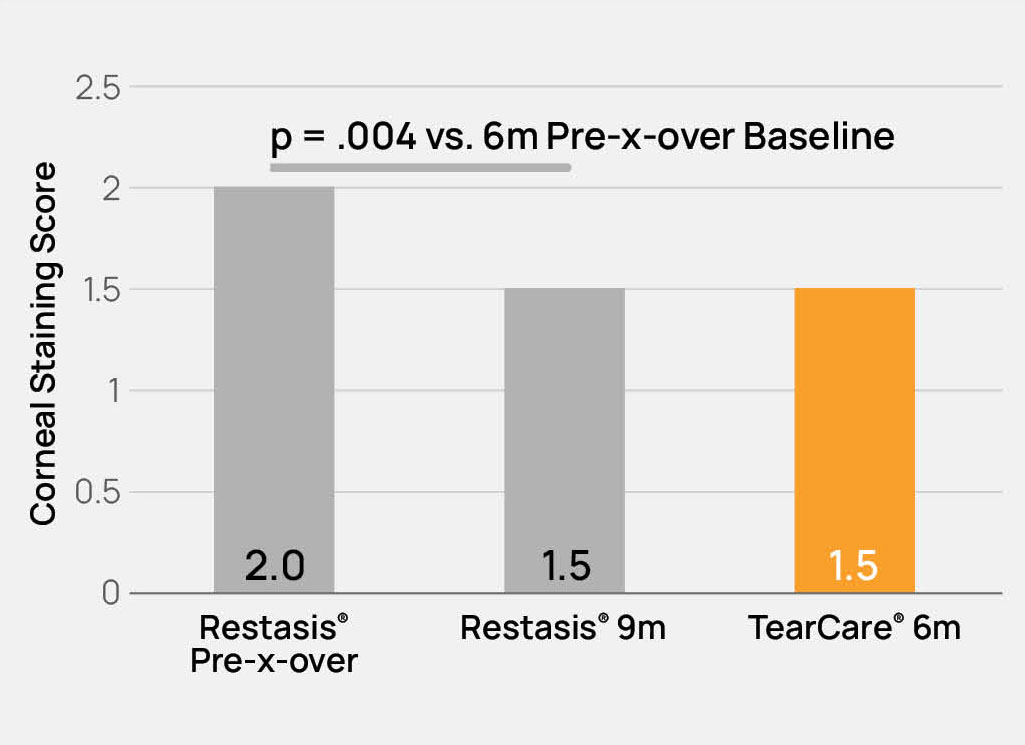
The TearCare® System is intended for the application of localized heat therapy in adult patients with evaporative dry eye disease due to meibomian gland dysfunction (MGD), when used in conjunction with manual expression of the meibomian glands.
CONTRAINDICATIONS
-
- The TearCare
®
- System is contraindicated for patients with:
- Recent (i.e. within the last 90 days) surgical procedure to the eye or eyelid.
- Recent ocular injury.
- History of Herpes Simplex or Herpes Zoster of the eye or eyelid.
- Active ocular or periocular infection, inflammation or irritation.
- Diminished or abnormal facial, periocular, ocular, or corneal sensation.
- Ocular surface ulcers.
- Hordeolum, stye, or chalazion.
- Do not use the TearCare® System in patients under the age of 22 years.
- Pacemakers or implantable cardiac defibrillators (ICD). Use of the TearCare® System may affect the performance of pacemakers or ICD’s due to electromagnetic interference (EMI). To avoid any potentially serious safety effects, patients with these implants should not be treated with the TearCare® System.
- Known allergy to acrylate.
- Known allergy to silicone tissue adhesives.
- Known allergy to copper.
WARNINGS
- Do not use the TearCare® System outside the instructions for use. Doing so can result in unanticipated patient harm.
- Do not attempt to connect the SmartHub™ or SmartLid™ directly to an electrical outlet of any kind.
- Do not use the TearCare® System in or near an MRI suite or near a magnetic field. Serious injury can occur to a patient or technician if a TearCare® System is brought into an MRI suite.
- The TearCare® System has not been tested in the presence of flammable anesthetics or other flammable agents in combination with air, nitrous oxide, or oxygen enriched environments.
- Do not apply SmartLids to non-intact skin (i.e., skin with active abrasion, cut, burn, rash, inflammation, redness, etc.)
PRECAUTIONS
- Use of the TearCare® System in patients with eyelid abnormalities (e.g., entropion, ectropion, tumor, edema, blepharospasm, lagophthalmos, severe trichiasis, severe ptosis, etc.) may result in poor adhesion of the SmartLid™ to the eyelid and/or reduced benefit.
- Use caution when using the TearCare® System in patients with ocular surface abnormalities (e.g. pterygium, pingueculum, corneal dystrophies, etc.) as the heat delivered by the System may aggravate these conditions.
- Remove contact lenses from the patient’s eyes prior to use of the TearCare® System. Patients should wait 60 minutes after the completion of the TearCare® procedure before re-inserting contact lenses.
- Do not apply the SmartLids™ to any other part of the patient’s body including the cornea. The SmartLids™ are only intended for application on the external surface of the patient’s eyelids.
- It is important for patient to keep their eyes open (blinking is permitted) during treatment, to allow heat to dissipate off of the ocular surface.
- Effectiveness of the TearCare®System has not been established in subjects for whom the treatment temperature is lowered from Warmth Level Setting #5 due to patient pain or discomfort.
- Do not reuse the SmartLids™. Cross-contamination can occur if re-use is attempted.
- Do not use a TearCare® System, its components, or accessories that appear damaged. Inspect all components for damage before each use.
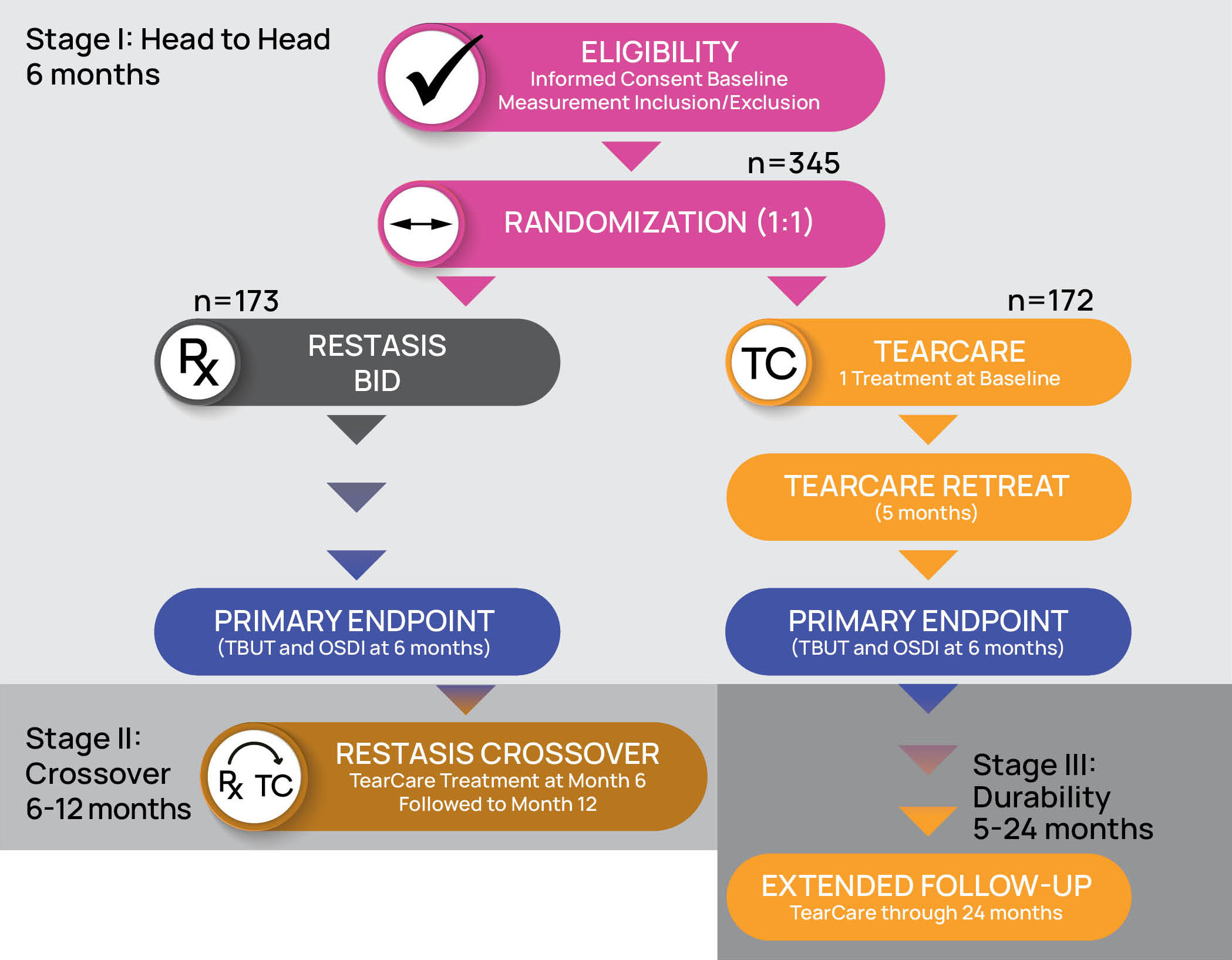
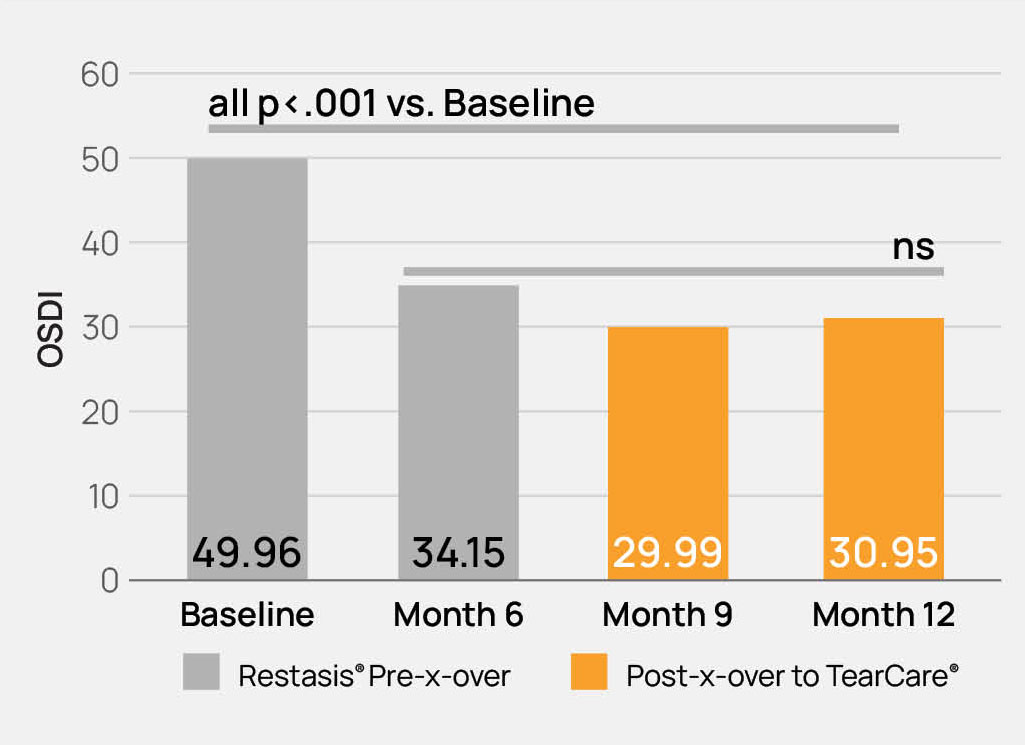
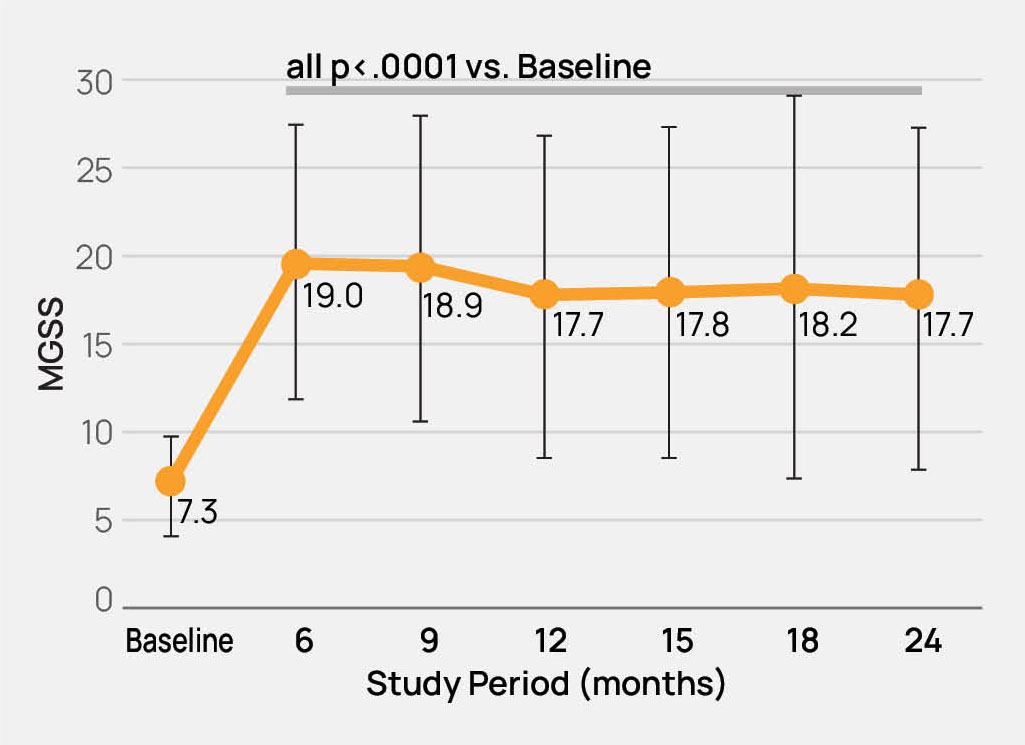
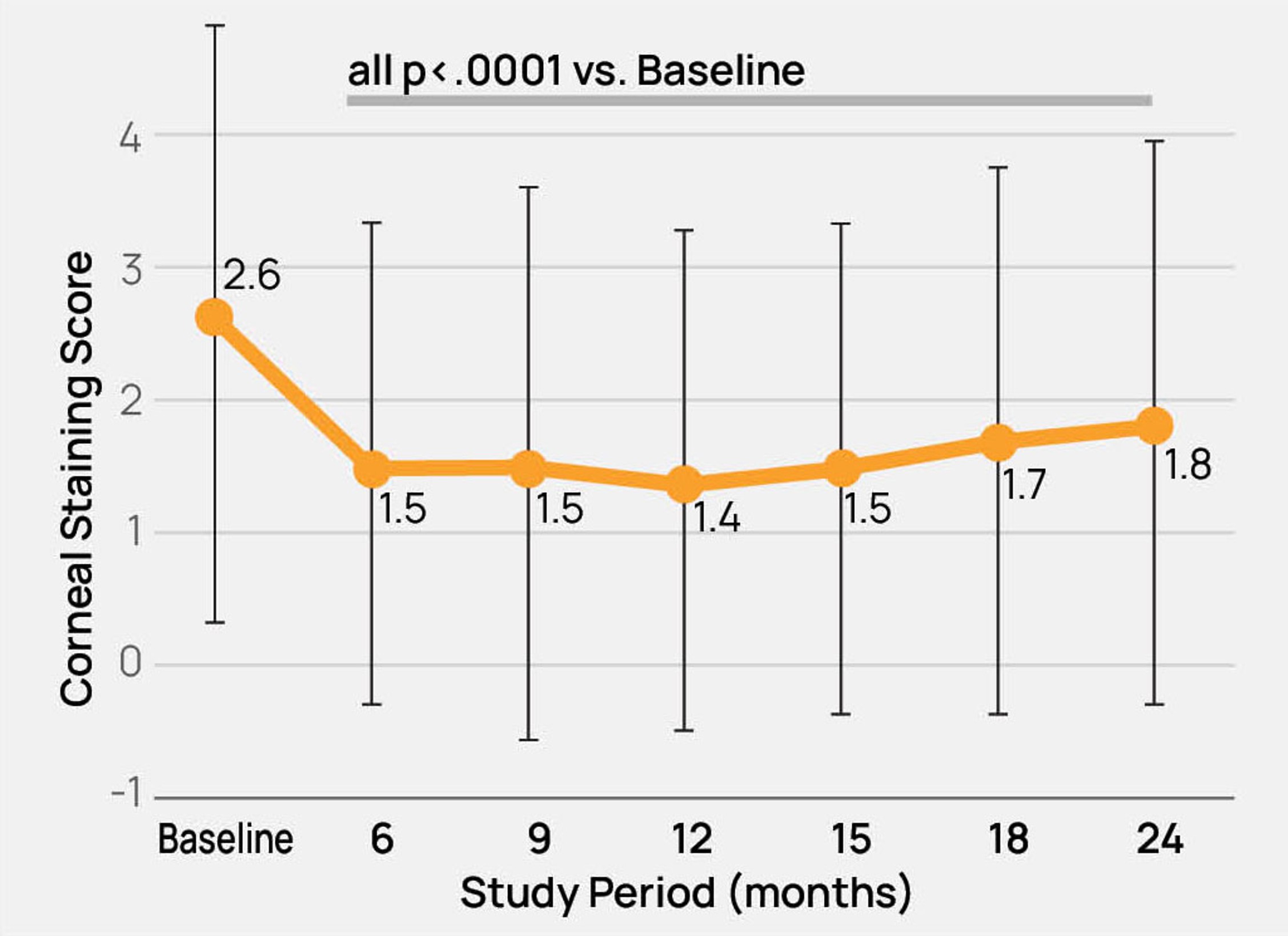
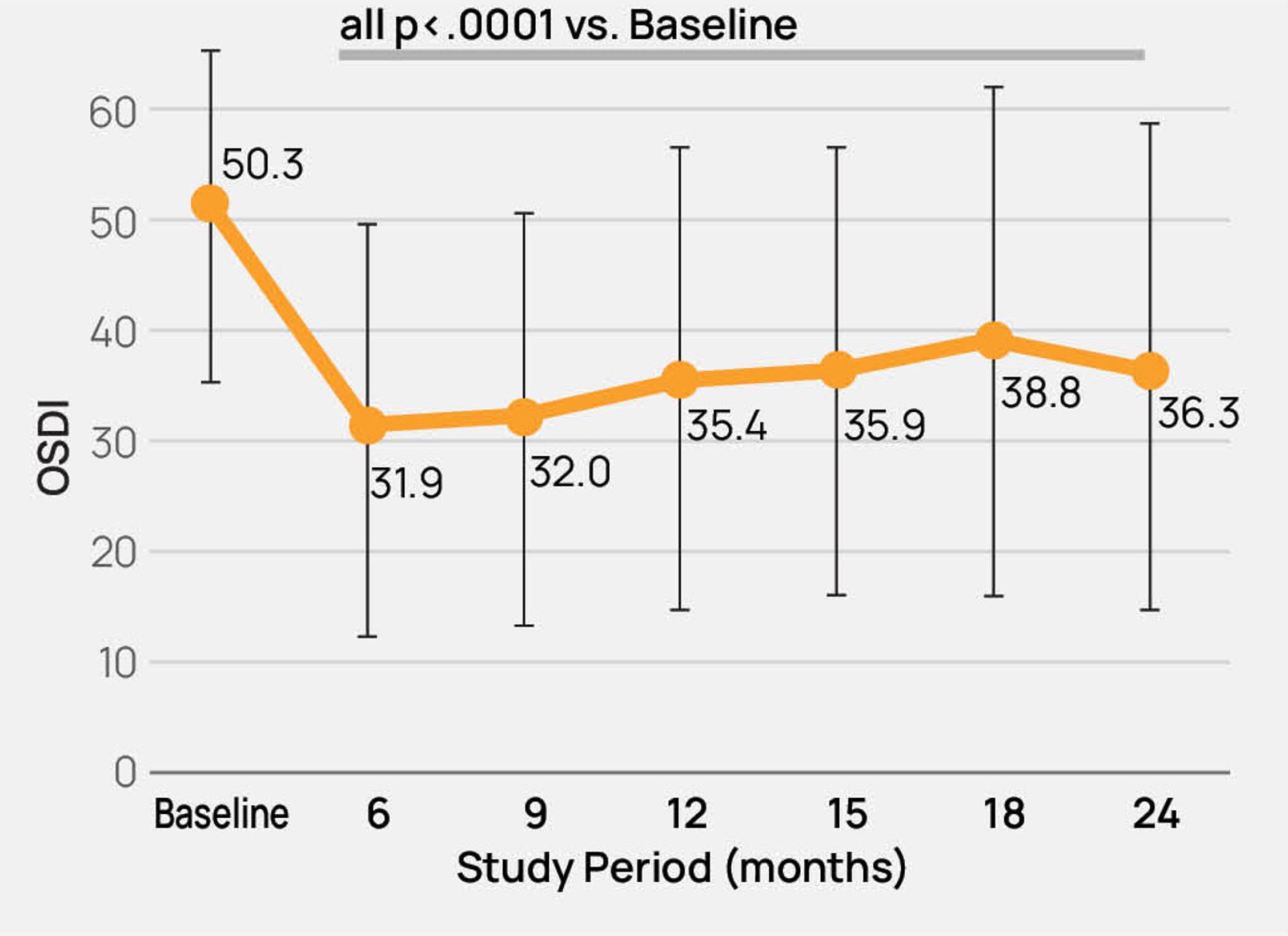
- The safety and effectiveness of the TearCare®System is not known in the following patient populations that were excluded in the OLYMPIA pivotal study: patients under 22 years of age, dry eye signs and symptoms other than meibomian gland dysfunction, severe signs and symptoms of dry eye due to meibomian gland dysfunction (OSDI > 79), mild signs and symptoms of dry eye due to meibomian gland dysfunction, and other study exclusions described in the TearCare®Instructions for Use.
POTENTIAL ADVERSE EVENTS
Potential adverse events may include but are not limited to:
- Eyelid or eye pain requiring discontinuation of the treatment procedure
- Eyelid irritation or inflammation*
- Ocular surface irritation or inflammation*
- Ocular symptoms (such as burning, redness, tearing, visual disturbance, redness)
- Burn, erythema, or swelling of the eyelids
- Conjunctival infection (moderate or severe)
- Conjunctival abrasion
- Corneal abrasion
- Corneal deformation
- Allergic or inflammatory reaction to medical adhesive on the SmartLid device
- Formation of a chalazion or stye*
- Decline in visual acuity*
- Worsening of dry eye symptoms*
- Increased discomfort or pain of ocular surface (grittiness, foreign body sensation, etc.)
- Discomfort or pain of eyelids or orbit*
- There is a potential risk of thermal injury to eye or eyelid based on the device design.